Bioorthogonal Ligations
Bioorthogonal Ligations
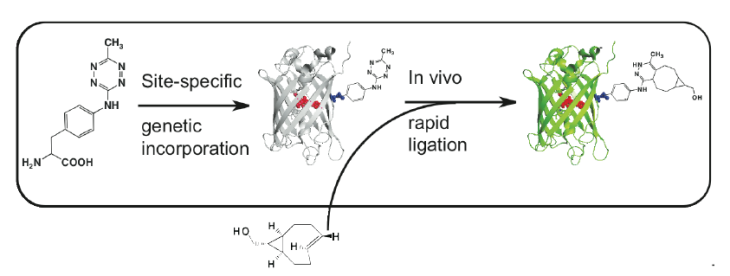
One of the most fascinating applications of bioorthogonal chemistry is the ability to site-specifically assemble biomolecules in live cells or whole organisms. Recently, a series of chemical reactions that are orthogonal to the functional groups within biological systems has revolutionized biochemistry; however, these reactions do not work well in cells or living organisms. Currently, the major limitations for ligations in living cells include the need for toxic metals, reagent stability, and the high concentrations of reagents needed to achieve reasonable reaction rates. To meet these demanding criteria, we have developed tetrazine/alkene ncAA coupling pairs that react quickly and orthogonally in living cells, allowing for temporal resolution of cellular processes. This “click type” chemistry for the attachment of proteins to small molecules, polymers, surfaces, or each other does not involve catalysts and will transform the core of biological materials science and engineering.
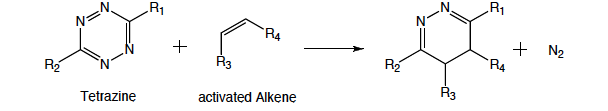
Figure 1. Altering the electron withdrawing and donating R groups can tune the tetrazine/alkene cycloaddition reaction rate to produce a stable cyclized product.
We have been successful in tuning the reactivity of the tetrazine/alkene pairs and evolving synthetases to utilize these optimized structures to produce bioorthogonal reactions with extremely fast reaction rates. (Genetically Encoded Tetrazine Amino Acid Directs Rapid Site-Specific in Vivo Bioorthogonal Ligation with trans-Cyclooctenes. J. Am. Chem. Soc. 2012, 134(6), 2898-901.)
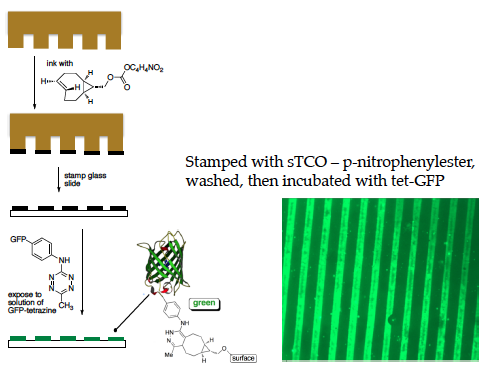
Using this ability to attach proteins site-specifically to any material we have labeled proteins in living organisms as well as patterned proteins directionally on surfaces.